

Ashley's journey through a clinical trial for atopic dermatitis was far from ideal. As a patient living with this condition, she bravely decided to participate, hoping to contribute to the development of new treatments and improve the lives of others like her. However, her experience fell short of expectations. Feeling unprepared and unsupported, Ashley's clinical trial left her feeling like just another number, rather than a valued partner in the process.
In her own words, Ashley shared, "I remember just through my clinical trial experience, it ended very abruptly, and I didn't know what my next steps were or how I was going to continue going on this medication. And so that left me, you know, feeling very isolated and alone and like, wow, I really did just feel like a number in this clinical trial."
This disappointing reality is one that many first-time clinical study participants face, leaving them feeling disconnected and unappreciated. Likewise, many study teams are nervous about the implications of treating patients like just another data point. They ask important questions about the risks this brings in failing to recruit patients for their study, losing patients to dropoff in the middle of the trial, or not winning consent for important substudies and data sharing that are essential parts of today's complex studies. How does treating patients like numbers cloud our views of how they experience trials, who should be in control, and how we should we relate to them?

In an ideal world, Ashley's clinical trial experience would have been a far cry from the disappointment and isolation she felt. Instead of feeling like just another number, she would have been welcomed as a valued partner in the research process from the very beginning.
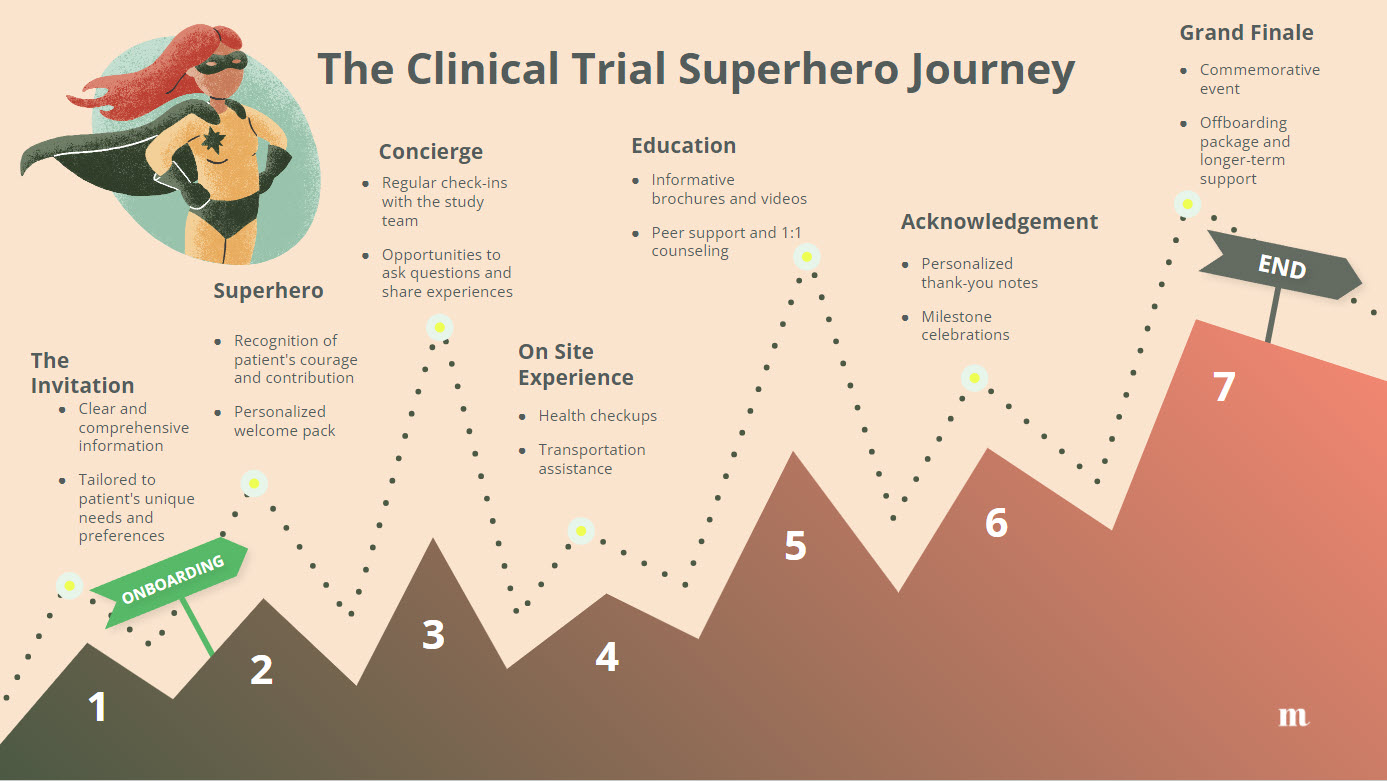

For Ashley, this reimagined clinical trial experience would have been a world apart from the abrupt and isolating reality she faced. Instead of feeling abandoned and uncertain about her future, she would have emerged from the trial feeling valued, supported, and empowered. Her journey would have been an example of what is possible when patients are placed at the heart of clinical research, and when their needs and experiences are given the attention and respect they deserve.
Ashley's story highlights the stark contrast between a traditional, patient-agnostic trial experience and the potential of a patient-centric approach. By reimagining her journey, we can see the potential of empowering patients as partners in the clinical trial process.
But how can we make this vision a reality? The answer lies in embracing co-design and patient mini-communities. By bringing together diverse groups of patients who are a good fit for a specific trial, researchers can gain valuable insights at every stage of the design process. Mini-communities allow for open and honest conversations about the challenges and concerns patients face, enabling study teams to create trials that are more engaging from enrolment through to completion.
Imagine how different Ashley's experience could have been if she had been part of a mini-community for her atopic dermatitis trial. As sorts of permutations are possible in co-design, but a simple two cohort approach would suffice. As a first-time trial participant, Ashley would have been invited to a cohort of trial inexperienced patients. By sharing her insights and collaborating with other patients, she could help shape the trial design to better meet the needs and expectations of participants and, in particular, addressing barriers to enrollment. Meanwhile, a second cohort would have consisted of AD patients with previous clinical trial experience. This cohort enables the study team to build on the real-life learnings of previous studies, reducing the risk of repeating others' mistakes and integrating opportunities to improve the study.
Through this co-design approach, the mini-community would enable a more comprehensive understanding of patient needs, ultimately resulting in a trial that is more engaging, inclusive, and effective. Ashley and her fellow participants would feel valued and heard throughout the process, knowing that their insights are making a real difference in the trial design. I know these are real benefits, and to be clear, when I say patients should be "partners," "co-designers," "collaborators," or "experts," I'm speaking metaphorically. Most patients don't have the scientific knowledge, regulatory understanding, or clinical expertise of researchers. So why take the risk? Because as imperfect as the analogy is, working with patients is easiest if you truly think of them as equal partners rather than passive participants or validators of your opinions.
As a patient experience strategist, I have seen firsthand how pharmaceutical teams benefit from involving patients as partners in various aspects of their work. By collaborating with patients, teams can gain valuable insights into the patient journey, identify unmet needs, and develop solutions that truly resonate with the patient community. Even teams with previous experience working with patients gain value with every round of patient collaboration, as patient needs are continually evolving in tandem with our own understanding of diseases and the treatment landscape.
To all the teams running clinical trials: it's time to take patient engagement to the next level. Don't just involve patients; embrace the power of mini-communities and co-design. By partnering with Merakoi and their innovative mini-community approach, you can unlock the full potential of patient insights and create trials that truly prioritize the needs and experiences of those who matter most.
To learn more about patient co-design in clinical trials, I recommend reading Dani Benson's insightful blog post, Unleashing Patient Power: Co-Design for Better Trials . Her guide explores the various aspects of patient involvement in clinical trial design and provides valuable insights for teams looking to adopt a more collaborative approach.
Contact us to discuss how to incorporate the voice of patients into your clinical trials.
About Merakoi
Merakoi partners with health and life sciences companies to build mini-communities that guide product development through continuous user insights. Our network of patients/advocates and proprietary community platform enable engaging, longitudinal co-creation between users and developers. The result is human-centered solutions that resonate powerfully in the real world.

✕