

From Passive Participants to Active Co-Designers
Imagine a world where clinical trials prioritize the needs of patients, not solely focus on testing new treatments. Co-designing with patients should be the starting point for clinical trial design, but sometimes their voices get lost in the shuffle. As the people who stand to benefit the most from new therapies, patients have a lot to say about what works and what doesn't. That's why it's so important to involve them from the very beginning.
When we invite patients to be active co-designers in clinical trials, we're doing more than just checking a box. We're recognizing that their experiences, opinions, and concerns are valuable and can shape the way a trial is run. By having open and honest conversations with patients, we can tackle issues that might make it tough for them to join or stay in a study. This could be anything from transportation problems to worries about the trial procedures or unknown results. By understanding each patient's perspective, we can make the trial experience better for everyone. When patients feel good about participating, it can lead to faster enrollment and better retention throughout the study.
But patient engagement is about more than just making trials run smoothly. It's also about building trust and creating a shared goal of improving healthcare. When patients feel like their voices are heard and valued, they're more likely to become champions for clinical research in their communities. This can greatly impact how the public sees clinical trials, breaking down misconceptions and encouraging more people to get involved.
Traditional market research and advisory board methods have long been the foundation of insight gathering for clinical trials. However, as technology and data rapidly evolve, it's crucial to recognize the limitations of these approaches and explore next-generation methodologies.
Market Research:
Advisory Boards:
While these methods have served us well, they may not be leveraging the full potential of advances in software, big data, and AI. It's time to embrace new approaches that address these limitations and unlock the power of continuous patient insights.
Imagine a solution that offers:
By adopting next-generation methodologies, we can revolutionize the way we conduct clinical research, leading to more effective, efficient, and engaging trials.
So, how can we make patient engagement easier and more effective? Enter the idea of mini-communities for your clinical programme. At Merakoi, our mini-communities bring together diverse patients who are the perfect fit for a specific trial, giving researchers a direct line to the people who matter most.
Let's say a research team is planning a trial for a new treatment for multiple sclerosis (MS). They could create a mini-community of MS patients with different experiences and backgrounds. This could include patients who have participated in trials before, those who are new to the process, and even patients from different geographic regions or with different types of MS.
By engaging with this mini-community, the research team can get valuable insights at every stage of the trial design process. They can ask patients for feedback on the trial protocol, making sure it's feasible and patient-friendly. They can test different recruitment materials and strategies to see what resonates best with patients. And they can even involve patients in designing the trial itself, getting their input on everything from the number of visits required to the types of assessments used.

One of the most powerful features of mini-communities is the ability to create cohorts, or subgroups, within the larger community. In our MS trial example, the research team could create cohorts based on patients' previous trial experience. They could compare insights from trial-savvy patients to those who are new to the process, identifying potential barriers and concerns for each group. This information can help the team tailor their approach to meet the needs of different patient populations.
Cohorts can also be used to gather insights on specific aspects of the trial experience. For example, the research team could create a cohort of patients who have used wearable devices in previous trials. By learning about these patients' experiences and preferences, the team can make informed decisions about incorporating wearables into their own trial design.
The value of cohorts in patient engagement cannot be overstated. By segmenting patients based on key characteristics or experiences, study teams can gain a more nuanced understanding of patient needs and preferences. This, in turn, allows for more targeted and effective trial design, ultimately leading to better recruitment, retention, and overall trial success.
Recently, I had the opportunity to work with a global clinical study team on enhancing a skin disease trial. As someone who has seen firsthand the challenges of patient recruitment and retention, I was excited to put the power of mini-communities and cohorts to the test.
We began by creating a mini-community of patients with the specific skin disease. Within this community, we established three main cohorts: patients who had previously participated in clinical trials and those who were new to the process, as well as a panel of patient advocates and expert. This allowed us to compare insights from trial-experienced patients with those who were trial-naive, giving us a more comprehensive understanding of patient perspectives.
One of the key issues we uncovered through our interviews with these cohorts was the impact of certain invasive procedures, such as skin biopsies, in the trial protocol. Trial-experienced patients were able to share their past experiences and concerns, while trial-naive patients expressed apprehension about these procedures. Patient advocates, though not necessarily the target population for the study, provided vital information on ensuring informed consent. By listening to these groups, we were able to work with the study team to refine the protocol, making it more patient-friendly without compromising scientific integrity.
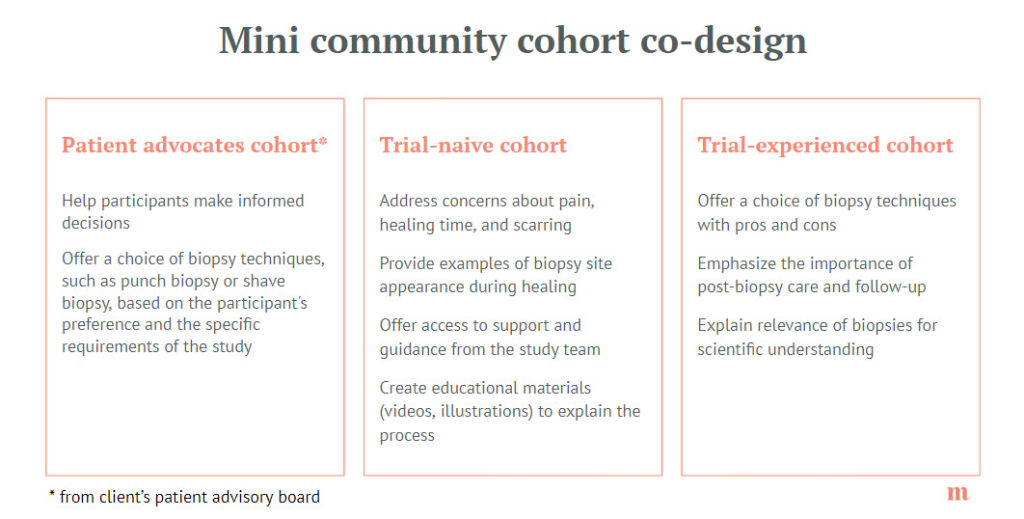
Another crucial aspect of our mini-community was its ethnic and geographic diversity. We made sure to include patients of different skin colors and from various national healthcare systems. This diversity proved invaluable, as we discovered that patients with darker skin tones often faced delays in diagnosis due to the difficulty in visually identifying symptoms. Patients from countries with more robust healthcare systems reported having better access to information and support networks, while those from regions with less developed infrastructure struggled to find the help they needed. This knowledge allowed the study team to tailor our recruitment materials and patient support programs to better meet the needs of patients from different backgrounds.
For patients in the mini-community around this clinical study, the experience is extraordinarily rewarding:
"You have to make the process comfortable for participants. They're spending their time doing this not only to help themselves but really to advocate for others because there's a lot of people that have this condition that don't have a voice. And what we are trying to do is help them come out of that. They don't have to be an advocate themselves, but they should advocate for themselves at least. And that's so important. You want the process to be smooth sailing and change from the way it has been, and it can change."
Ashley Wall, Merakoi Patient Expert
At the end of the day, a successful clinical trial isn't just about the scientific results. It's also about making a real difference in patients' lives. By putting patients at the center of the process and working together to design better trials, we can unlock the full potential of clinical research. This means faster development of life-changing treatments and a brighter future for healthcare, one trial at a time.
For a comprehensive guide on patient engagement in clinical trial co-design, check out this valuable resource from PFMD: Patient Engagement in Clinical Trial Protocol Design (Merakoi is a contributor)
Ready to change the way we do clinical research? Contact us for more information about mini-communities.
About Merakoi
Merakoi partners with health and life sciences companies to build mini-communities that guide product development through continuous user insights. Our network of patients/advocates and proprietary community platform enable engaging, longitudinal co-creation between users and developers. The result is human-centered solutions that resonate powerfully in the real world.

✕