

Effective Patient-Reported Outcome (PRO) measures in clinical trials can provide a significant competitive advantage for pharmaceutical companies. Demonstrating improvement in key symptom domains that competitors have not proven offers a powerful differentiation in the market, attracting both patients and prescribers to new treatment options. By investing in robust PRO development and implementation, companies can strengthen their product positioning and drive commercial success.
In the pursuit of patient-centred healthcare, harnessing the wisdom and expertise of people living with multiple sclerosis (PwMS) is crucial to drive transformative change. New MS treatment options have successfully shown reduced functional impairment through fewer disability-worsening events, which is seen by PwMS as a major achievement. In pivotal clinical trials, multiple PRO measurements are being included to understand the impact of treatment on other symptoms like Fatigue, Cognition, Pain, and Depression.
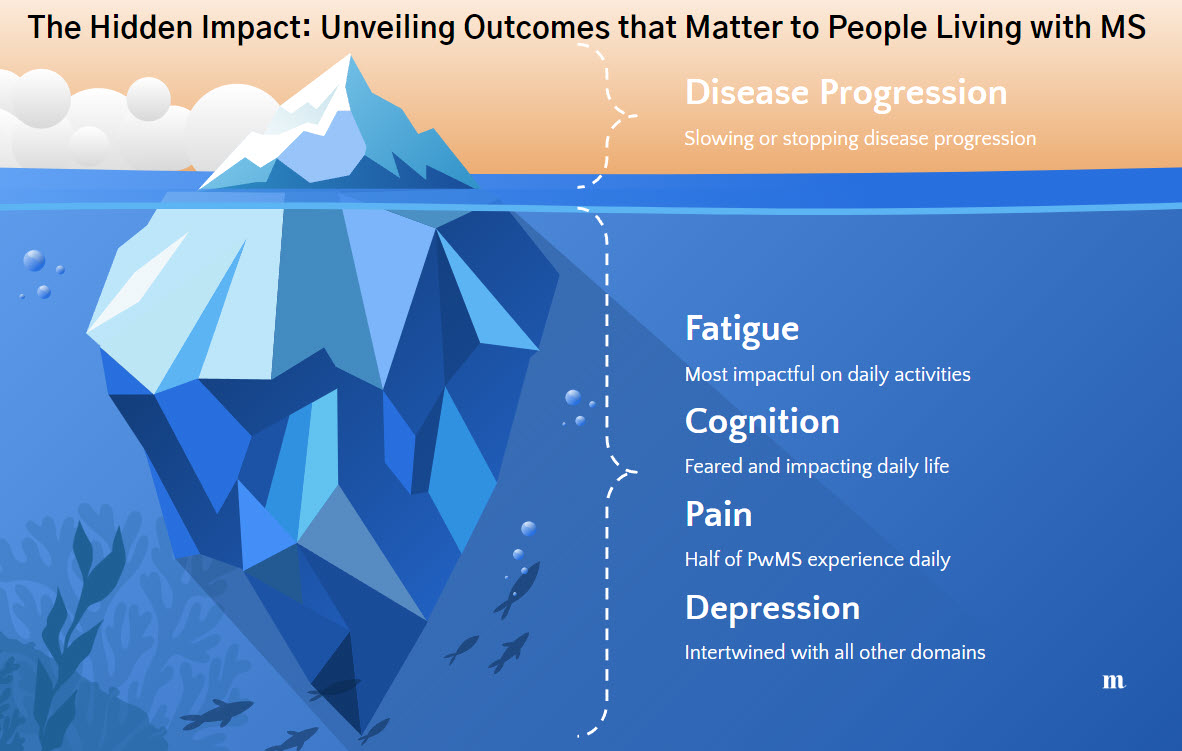
However, there is doubt about the robustness of some of these tools, raising concerns about the true meaningfulness of the data obtained. For PwMS, those 4 domains provide major challenges, and any treatment that would impact these symptoms is highly welcomed.
To assess the alignment of 6 PROs with regulatory and scientific requirements on PRO structure/development, under the leadership of merakoi a diverse and representative panel of PwMS evaluated the degree to which the PROs reflect disease aspects they perceive as important. A patient-led qualitative market research was performed to gain insights and recommendations from PwMS, better understand the impact of those 4 domains on their daily life and evaluate the degree to which the 6 most used PROs in MS pivotal trials reflect disease aspects they perceive as important.
Our research journey uncovered invaluable insights from PwMS, illuminating the complex realities of living with this chronic condition. Here's a closer look at the outcomes:
Fatigue: Beyond Physical Exhaustion
MS-related fatigue emerged as a pervasive challenge impacting daily life. It is always present and unpredictable, so it affects normal day-to-day activities. PwMS emphasized the need for PRO measures that capture the diverse dimensions of fatigue, including cognitive and emotional impacts, to inform more effective interventions.
Cognition: Navigating Cognitive Challenges
Cognitive impairment in MS presents varied manifestations. The progression of cognition is feared, impacting many areas from daily activities to a sense of purpose in life. Patients emphasized the importance of tailored assessments to capture nuances in cognitive function, guiding the development of more precise measurement tools.
Pain: Understanding the Spectrum of Discomfort
Pain comes not from one, but multiple sources. It is usually invisible and half of the PwMS interviewed experience pain daily. Pain in MS spans a spectrum of sensations. PwMS emphasized the need for personalized pain management strategies, supported by PRO measures that account for the subjective nature and varied types of pain experienced.
Depression: Addressing Mental Health Needs
Depression is highly interconnected with the other domains (and is often exacerbated by them) and in many cases requires professional intervention. Depression is a significant comorbidity in MS, impacting emotional well-being. Patients stressed the importance of PRO measures that assess emotional distress comprehensively, facilitating holistic care approaches. Realizing that depression is not all the time experienced but can come in waves.
To uncover these rich patient insights, merakoi employed a unique methodology that empowered PwMS as research partners:

By harnessing the power of patient-led research within an engaged community, unparalleled access was gained to the real-world experiences, needs, and priorities of PwMS. These insights can inform the refinement of PRO measures and equip pharma partners with an evidence-base to enhance clinical trials and product development.
Building on these insights, here are strategic recommendations for health companies seeking to integrate patient perspectives into their innovation processes:
Ready to infuse patient perspectives across your product lifecycle? Merakoi can help.
From early-stage development to clinical trials and commercialization, Merakoi provides the patient-generated evidence and insights needed to create truly patient beneficial health innovations. Our unique mix of patient expertise, agile research methodologies, and scientific rigor helps optimize PROs, enhance trial design, and build compelling value propositions. Merakoi can overcome the challenges associated with patient engagement e.g. identifying, educating and compensating patients and patient experts at fair market values.
Partnering with merakoi means:
Don't miss the opportunity to differentiate your pipeline. Contact us to discuss how to incorporate the voice of patients into your programs.
About Merakoi
Merakoi partners with health and life sciences companies to build mini-communities that guide product development through continuous user insights. Our network of patients/advocates and proprietary community platform enable engaging, longitudinal co-creation between users and developers. The result is human-centered solutions that resonate powerfully in the real world.

✕