
Today, May 20th, we celebrate International Clinical Trials Day, a commemoration of the day that James Lind laid the foundation for modern clinical research in May 1747.
At a time when scurvy was thought to have killed more British seamen and sailors than French and Spanish armies combined, surgeon’s mate James Lind conducted what many refer to as the first clinical trial. Lind selected 12 scurvy-stricken men from the ship. They were divided into six pairs, with each group receiving a different addition to their basic diet. Some were given cider, others seawater, or vinegar. And the last group, oranges, and lemons, Those fed citrus fruits experienced, in the words of Lind “the most sudden and good visible effects.”
Fast forward to today, and the conduct of a clinical trial is a rigorous process involving multiple phases, from preclinical to real-world evidence after the intervention is approved by regulators. Clinical trials and their various phases constitute a vital part of clinical research. In this way, new drugs and treatments can be evaluated for safety and effectiveness before they can be approved for general use.
In early phase development, understanding the patient target population requires a coordinated effort to design a trial that will lay the foundation for later-stage success. Timely recruitment of patients is widely recognised as an essential component of successful clinical trials. However, the clinical research industry faces a significant problem with delays in patient recruitment. Current estimates show that 80% of clinical trials miss their patient enrollment deadlines. In turn, this impacts how quickly new medicines can reach the patients who need them.
In order to better understand the knowledge and attitudes of patients towards early phase clinical trials, we conducted a survey among our patient experts.
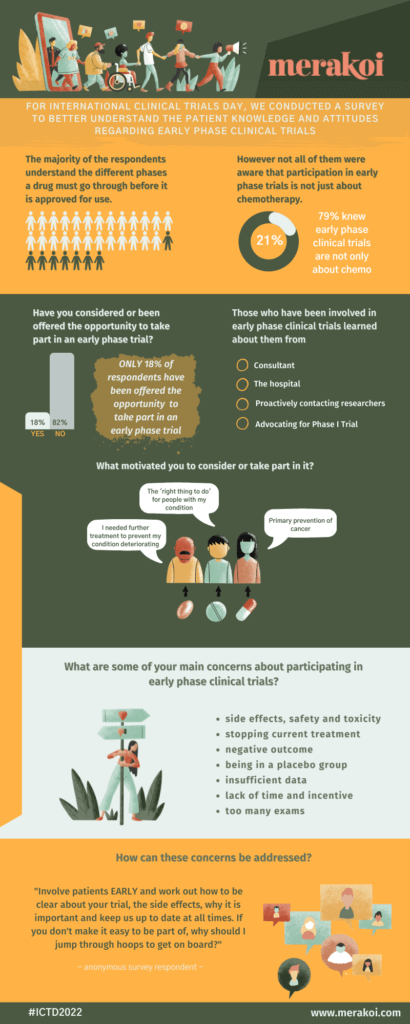
The majority of survey respondents (76%) understand the different phases a drug must go through before it is approved for use.
Just 18% of respondents have been offered the opportunity to take part in an early phase trial. When asked where they had heard about the trial, respondents mentioned the following sources:
Respondents provided the following reasons for taking part:
A majority of respondents (79%) knew that participation in early phase trials can give access to interventions beyond chemotherapy.
When asked to elaborate on concerns regarding their participation in early phase clinical trials, respondents gave the following reasons:
Finally, we asked respondents how these concerns could be addressed to allay patient fears.
“I have taken part in several Phase I early Phase II trials - but only if I can be a 'member of the team' and kept fully up to date. I WILL NOT take part if I am just considered a human-shaped lab rat. So! Teams! Get your act together, involve patients EARLY and work out how to be clear about your trial, the side effects, why it is important (Is it important, or just a PhD/vanity project??) and keep us up to date at all times. If you don't make it easy to be part of, why should I jump through hoops to get on board?” - - - anonymous survey respondent
Optimising early clinical trials involves distinct phases focused on understanding patients, the target populations, their needs, and how to reach them, including better education and ongoing and pertinent communication. Sometimes programnes that understand these fundamentals are needed for patient behavioural change if retention or recruitment issues already exist.
Generally, clients work with KOLs and investigators to finalize the target patient population and trial objectives for early trials in order to move the trials quickly to the next phase—the "fail and fail fast’ attitude. Budgetary allocations rarely cover patient input. This can be a costly mistake in delaying the overall clinical development program. Direct validation with patient experts and understanding the emotional and practical aspects of considering or participating in a trial—such as choices, attitudes toward participation, risks, and fears—is another aspect that clients will not gain unless they also hear directly from patient experts and their diverse patient networks.
We must consider ways to increase awareness of early phase clinical trials - where do patients go to find out about all the ongoing trials, and what are the pain points (barriers to entry) for them?
To maximise recruitment for an event like this, all trial touchpoints need to be validated and thought through, including how to describe trial materials and patient participation materials to ensure maximum uptake and retention. It will help patients and caregivers recognise that this is for them or someone close to them.
So if you are embarking on a early phase clinical trial and you want to hear more about involving patient experts to ensure success, get in touch with merakoi through Helen Hey.

✕